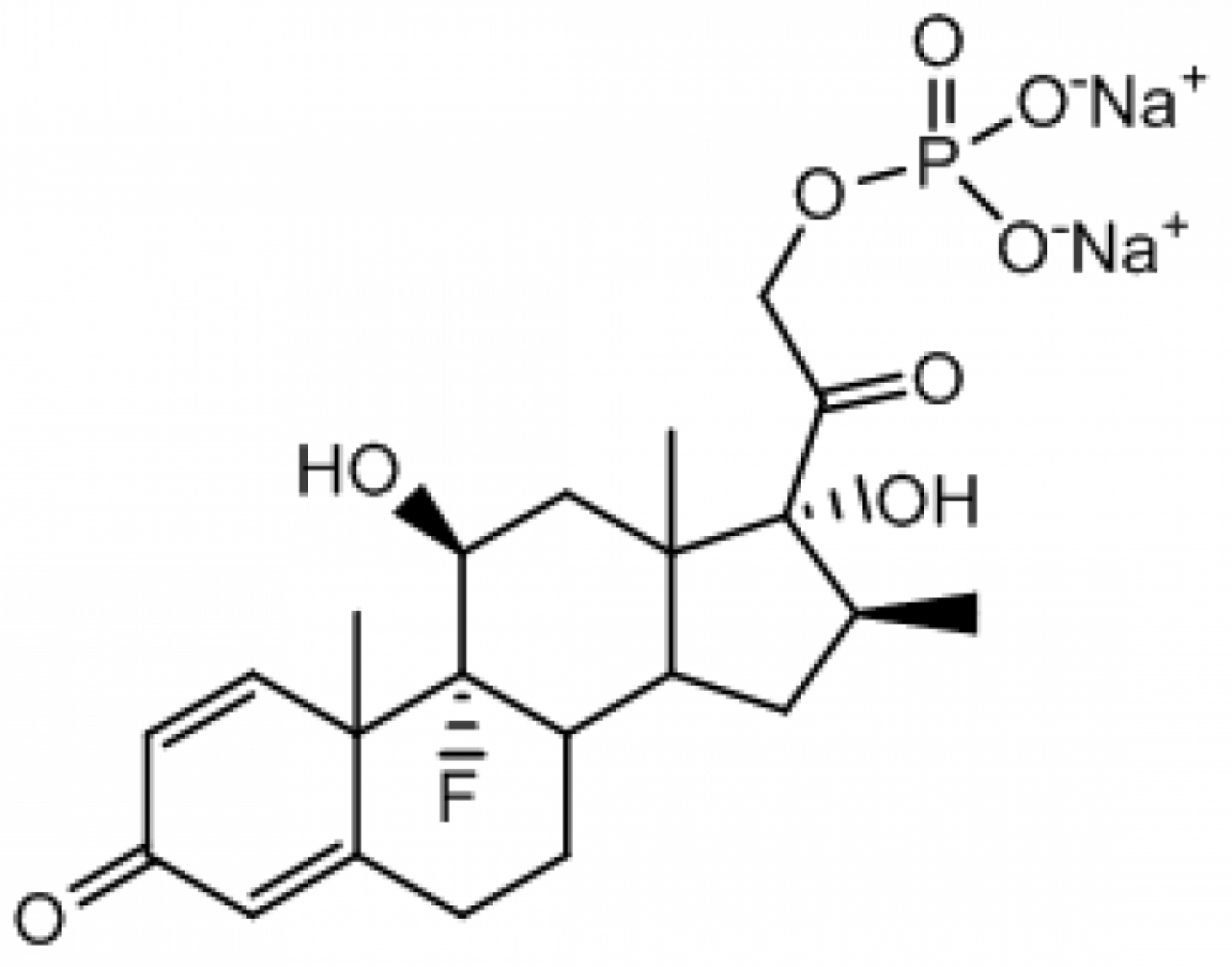
Betamethasone Disodium Phosphate
API Product
Product Status:
Commercial
Available Grades:
- 粉砕品
Regulatory Status:
日本 DMF
米国 DMF
Production Sites:
- Hovione Loures(ポルトガル)
Product Type:
- コルチコステロイド
CAS Number:
151-73-5
Modes of Application:
- 外用
- 注射
- 点眼
Common Indications:
- アレルギー反応
- 経口剤
Last Inspection:
FDA May 2018
Hovione produces Betamethasone Sodium Phophate since 1980 and has over 50 years experience in the manufacture of corticosteroids.
Injectable quality available on demand from the European producer with an unblemished Regulatory track record.
Approved in branded and generic human applications.
This is not to be construed as a representation of non-infringement or as an offer to sell in those countries where such would constitute an infringement of third parties' patent rights.
Highlight